Abstract
Introduction: Glomerular and tubular dysfunctions in β-thalassemia major (β-TM) patients (pts) have been attributed to iron overload, chronic anemia and iron-chelation therapy (ICT) toxicity. We studied glomerular and tubular function in β-TM pts treated with 2 different ICT regimes.
Patients and methods: We studied 36 β-TM pts (18 females and 18 males). Mean age was 20.92 ± 9.7 (range 5-45) years. Pts were treated at Emek Medical Center under standard protocols for regular blood transfusions and ICT: 26 pts received deferasirox (DFX) for a mean period of 59 months; this was the first ICT for 6 of them; 10 pts were treated with deferoxamine (DFO) or DFO + deferiprone (DFP) (4 and 6 pts, respectively; hereafter DFO+/-DFP group). Clinical data were collected from pts' files.
We evaluated serum urea, creatinine (sCr) and electrolytes, and estimated glomerular filtration rate (eGFR) according to the Schwartz formula for pediatric pts and the Chronic Kidney Disease Epidemiology Collaboration formula for adults; fractional excretion of sodium (feNa), fractional excretion of potassium (feK), calcium-to-creatinine ratio (Ca/Cr), uric acid excretion (UAE), and tubular phosphorus reabsorption were calculated. Urinary N-acetyl-β-D-glucosaminidase (uNAG) was also measured as a marker of tubular injury.
Results: No significant differences were found in age, gender or mean hemoglobin (Hb) level between the groups. Levels of sCr, K, Na and feNa were in the normal range for all pts, as was eGFR (mean 104.6 ± 19 mL/min per 1.73 m2); in the DFX group, eGFR was slightly lower than in the DFO+/-DFP group (100.9 ± 17.09 vs. 114.0 ± 22.31 mL/min per 1.73 m2; p= 0.0676). Hypercalciuria (Ca/Cr > 0.25) was found in 28% of pts, increased feK (>15%) in 33%, high UAE (>0.56 mg/dL) in 64%, and high levels of TmP /GFR (>5mg/dL) in 25%. There were no differences in these parameters between the DFX and DFO+/-DFP groups.
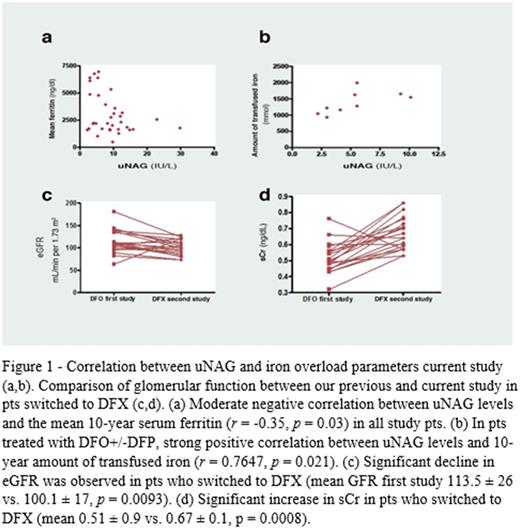
Increased uNAG was found in 9 pts (25%)-30% in the DFX group vs. 10% in the DFO+/-DFP group, and was significantly higher in the DFX group (mean 10.4 ± 6.1 vs. 5.3 ± 2.7 IU/L, p= 0.012). A moderate negative correlation was found between uNAG levels and mean serum ferritin for the prior 10 years (r= -0.35, p= 0.03). In the DFO+/-DFP group, a strong positive correlation was found between uNAG levels and the amount of iron transfused over the prior 10 years (r= 0.7647, p= 0.021); this correlation was not found in DFX pts. A moderate negative correlation was found for urinary Ca/Cr ratio with 10-year mean serum ferritin level and with 10-year amount of transfused iron (r= -0.35, p= 0.34; r= -0.4, p= 0.014, respectively). These correlations were stronger in the DFO+/-DFP group. A positive correlation was found between urinary Ca/Cr ratio and Hb levels, strongest in the DFO+/-DFP group than the DFX group (r= 0.41, p= 0.001, r= 0.7, p= 0.018), respectively.
Renal function had been previously evaluated in 20 pts treated with DFO (Smolkin et al, 2008) and those results were compared with the current values. The eGFR significantly declined in pts switched to DFX (mean eGFR in first study 113.5 ± 26 vs. 100.1 ± 17 mL/min per 1.73 m2, p= 0.0093) but not in pts who continued DFO+/-DFP. A significant increase in sCr compared to the previous study was found in pts who switched to DFX (mean 0.51 ± 0.9 vs. 0.67 ± 0.1 mg/dl, p= 0.0008), but not in pts who continued treatment with DFO+/-DFP. The same observation was made regarding urine Ca/Cr (mean 0.08 ± 0.11 vs. 0.176 ± 0.12, p= 0.001).
Conclusion: Ahigh prevalence of renal tubular abnormalities was observed in our pediatric and adult β-TM pts, particularly in the DFX group. The marker of tubular injury-uNAG-was negatively associated with mean 10-year serum ferritin, suggesting ICT's involvement in tubular injury. Moreover, uNAG was associated with transfusional iron burden in the DFO+/-DFP group, but not in the DFX group, proposing a mechanism other than iron overload for the pathogenesis of tubular injury in DFX-treated pts. Finally, glomerular function remained within the normal range in all pts; however a significant decline in glomerular function compared to a decade earlier was observed only in the pts currently treated with DFX. Strict follow-up of renal function in β-TM pts, especially children, is warranted.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal